Combustion of Butane in Excess Oxygen
Butane CAH10 undergoes combustion in excess oxygen to generate gaseous carbon dioxide and water. Please correct in the notes page 39.

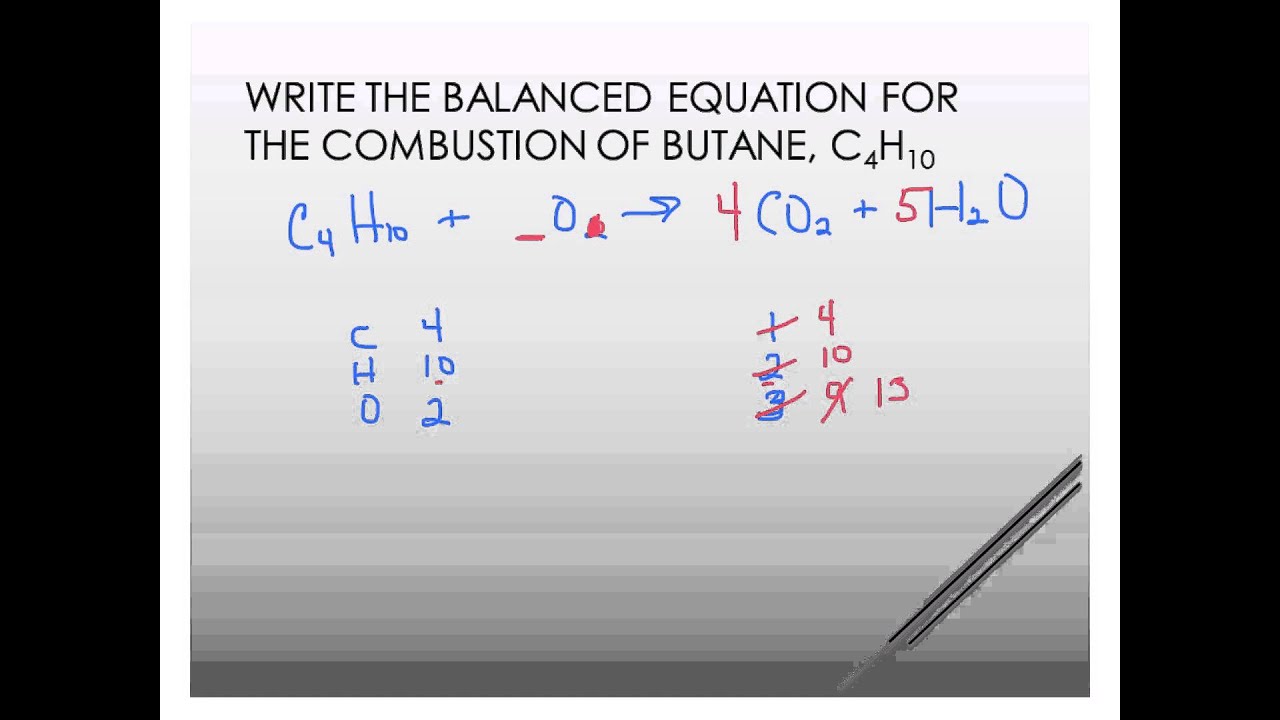
Complete Combustion Of Butane C4h10 Balanced Equation Youtube
The chemical equation for stoichiometric combustion of methane - CH 4 - with air can be expressed as.
. Complete combustion does NOT give carbon monoxide or sootCheck me out. Unlike natural gas propane is heavier than air 15 times as dense. Butane C4H10 reacts with oxygen O2 to make carbon dioxide CO2 and water H2O.
Write the combustion product of butane in excess oxygen. Butane C4H10 Reacts With Oxygen O2 To Form Water H2O And Carbon Dioxide CO2 As Shown In The Following Chemical Equation. To create a proper balance one may only adjust the numbers of one product or one reactant at a time.
Butane c4h10 undergoes combustion in excess oxygen to generate gaseous carbon dioxide and water. Match the following items. If pure oxygen were to be supplied the rate of combustion would increase significantly.
High-intensity bundle of energy being emitted from some decaying nuclei gamma ray 2. Calculate the energy in kJ produced when 500 g octane is burned in excess oxygen. What formed when butane burns in excess air.
Carbon dioxide and water. This may occur in several ways either by oxidation in the presence of. Assuming the CO level in flue gas is very low and incomplete combustion can be neglected oxygen content in flue gas can be measured in two numbers.
The following reactions are not balanced but we need the stoichiometric coefficient of butane to be -1 in both reactions 1 C4H10 1 C4H10 02 -CO2 -H20 Reaction 1 02 -CO -H20. 2C4H10 g13O2 g10H2O g8CO2 g Calculate The Mass Of Butane Needed To Produce 715 G Of Carbon Dioxide. Chemistry questions and answers.
Butane C4H10 is a common fuel used for heating homes in areas not served by natural gas. How would you remove the 132 coefficient for dioxygen. The chemical formula of butane is C4H10.
Carbon dioxide and water. With those measurements the following formulas can be used to calculate excess air. On the other hand 031 mol of O2 requires 213x031 0048 mol of C4H10 for a complete combustion to produce the said products.
What products are formed when butane burns in excess air. 2C4H10 1302 8C02 10H2O 44 molecules 265x1024 molecules 44x1023 265x1024 formula units. Then there is more energy given off when 10 g of butane burns in pure oxygen rather than in air.
Particle radiating from the nucleus of some atoms beta particle 3. Butane C4H10 is burned completely in excess oxygen write the balanced chemical formula for the reaction name the products and type of reaction. C4H10g132O2g4CO2g5H2Og Is the equation above balanced.
What are the reactants and products when butane combusts with excess oxygen chegg. CH 4 2O 2 376 N 2 - CO 2 2 H 2 O 752 N 2. The number of molecules of carbon dioxide produced by the combustion of 11 mol of butane C4H10 in presence of excess oxygen is.
Butane C4H10 burning in air is an example of a substance combining with oxygen to produce CO2 and H2O. 200 theoretical air is 100 excess air. The chemical formula of butane is C4H10.
2C4H10g 13O2g 8CO2g 10H2O g Answer link. Multiple Choice 2910 kJ 379 kJ 759 kJ 22300 k. - how many grams of butane can be burned by 254 moles of oxygen.
The additional air is termed excess air but the term theoretical air may also be used. Given Hf C4H10g -1247 kJmol HfCO2g -3935 kJmol HfH2Og -2418 kJmol how much energy is released kJ when 830 g of butane is burned. Get the answer to your homework problem.
3 Get Another question on Chemistry. What can cause spontaneous combustion. The quantity of oxygen theoretically required to ensure complete combustion As the composition of air is known the Theoretical or Stoichiometric Air can easily be calculated by multiplying the stoichiometric oxygen by the molar ratio of 10021 ie.
The combination produces eight molecules of carbon dioxide and 10 water molecules. In its unbalanced form the equation for the combustion of. Butane oxygen carbon dioxide water Explanation.
The combustion of butane is a reaction between butane and oxygen gas that produces carbon dioxide gas and water. Therefore the butane is in excess of 017-0048. What happens when butane is burned in excess of oxygen.
Dry reading A or wet reading B. In the presence of excess oxygen propane burns to form water and carbondioxide. Spontaneous combustion can occur when a substance with a relatively low ignition temperature hay straw peat etc begins to release heat.
C 4 H 10 O 2 ---- CO 2 H 2 O. The heat of combustion of octane is -552E3 kJmole. 2C 4 H 10 g 13O2g 8CO 2 g 10H 2 Og.
Butane C4H10 undergoes combustion in excess oxygen to generate gaseous carbon dioxide and water. The combustion of butane is a reaction between butane and oxygen gas that produces carbon dioxide gas and water. All parts of this question are related to this reaction.
Given AHC4H1olg - 1247 kmol AHCO29 -3935 kJmol AHH20g -2418 kJmol how much energy is released kJ when 830 g of butane Is burned. The equation for its combustion is 2C4H10 13O2 -- 8CO2 10H2O. The combustion of butane in 20 excess oxygen at atmospheric pressure produces CO2 and H2O in one reaction and CO and H20 in the second reaction.
The combustion of butane is a reaction between butane and oxygen gas that produces carbon dioxide gas and water. Negative particle identical to. For example if the oxygen dry reading in flue gas is 25 then the excess-air calculation.
Butane oxygen carbon dioxide water. If more air is supplied some of the air will not be involved in the reaction. The reaction above represents complete combustion.
The balanced equation for the combustion of butane combines two molecules of butane with 13 oxygen molecules. How do you know. This is the basic UNBALANCED equation.
When not enough oxygen is present for complete combustion incomplete combustion occurs when propane burns and forms water carbon monoxide carbon dioxideand carbon. Given δhf c4h10 g 1247 kjmol δhf co2 g 3935 kjmol δhf h2o g 2418 kjmol how much energy is released kj when 830 g of butane is burned. We need to think about the number of atoms of each element in the reactants and ensure these are the same in the products.

How To Balance C4h10 O2 Co2 H2o Butane Combustion Reaction Youtube

Solved Butane C4h10 Undergoes Combustion In Excess Oxygen Chegg Com

Solved 3 The Combustion Of Butane Is Represented In The Chegg Com

Position Isomers Chain Isomers And Functional Group Isomers Functional Group Positivity Molecules

Solved Part B Calculate The Mass Of Water Produced When 4 06 G Of Butane Reacts With Excess Oxygen Express Your Answer To Three Significant Figures And Include The Appropriate Units View Available Hint S
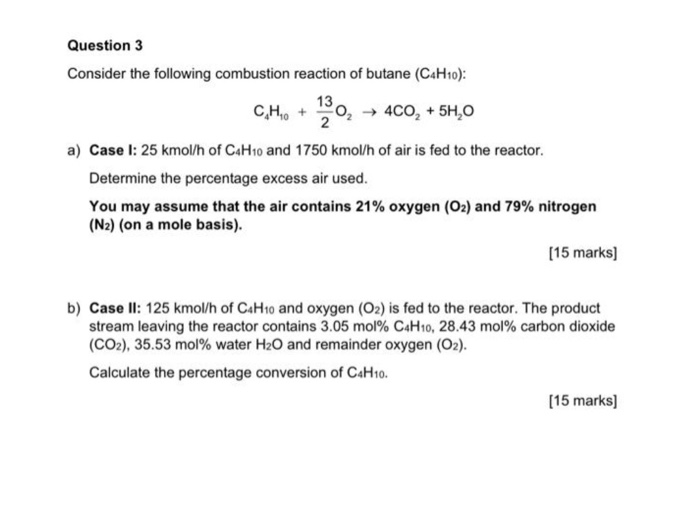
Solved Question 3 Consider The Following Combustion Reaction Chegg Com

Solved The Combustion Of Butane In 20 Excess Oxygen At Chegg Com

Solved Here Is The Reaction Equation For The Combustion Of Butane Which Is An Exothermic Reaction 2 Cahio G 13 Oz G 8 Coz G 10 Hzoka Which Of The Following Will Produce

Balance Combustion Butane 20pct Excess 85pct Consumed Youtube

Solved Butane C4h10 Burns In Oxygen To Make Co2 And H2o Given Excess Oxygen How Many Grams Of Co2 Does The Combustion Of 80g Of Butane Make

Solved Consider The Following Combustion Reaction Of Butane Chegg Com

Solved Back 9 13 Limiting Reagents And Perce Ij What The Balanced Equation For The Reaction Of Butane Chhjo With Oxygen 0 T0 Produce Carbon Dioxide Co And Talet H Oj This Called Combustion Rezction

Calculate The Mass Of Water Produced When 2 50 G Of Butane Reacts With Excess Oxygen Homeworklib

When C3h8 Is Burned In Oxygen The Products Are Lisbdnet Com

Hydrogen Fuel Cell High School Hydrogen Fuel Cell Hydrogen Generator Fuel Cell

The Combustion Of Alkanes Organic Chemistry Visual Carbon Dioxide

When 1 3 G Of Butane C 4 H 10 Was Burnt In Oxygen In A Flame Calorimeter Containing 1 8 Kg Of Water The Temperature Reose From 25 3 To 33 3 C Calculate The Enthalpy Change For The Combustion Of 1 Mol Of Butane

Comments
Post a Comment